Magnesium Chloride and Sodium Hydroxide Net Ionic Equation
When two solutions of ionic compounds are mixed a solid may form. Mg 2 aq 2 NO 3 - aq Ca 2 aq 2 Cl - aq -- Ca 2 aq 2 NO 3 - aq Mg 2 aq 2 Cl - aq Net Ionic Equation.
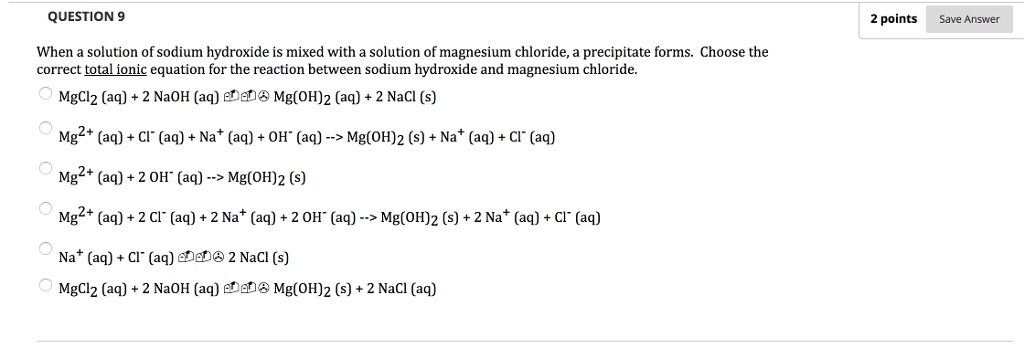
Solved Question 9 2 Points Save Answer When A Solution Of Chegg Com
Magnesium chloride react with sodium hydroxide to produce magnesium hydroxide and sodium chloride.

. The chemical equation for the reaction of magnesium chloride and sodium hydroxide is given as. If playback doesnt begin shortly try restarting your device. Honors Chemistry Name_____ Period_____ Net Ionic Equation Worksheet READ THIS.
Mg Cl 2aq 2 Na OH aq Mg OH 2s 2 Na Cl aq The ionic equation is Mg 2aq 2 OH -aq Mg OH 2s M agnesium hydroxide is white. Be sure to consider the solubility of products formedthis will help identify spectator ions MgCl 2 aq 2NaOH aq Mg OH 2 s 2NaCl aq Mg 2 aq OH aq MgOH s. Write balanced molecular equation and net ionic equations for the following reactions.
First we balance the molecular equa. Write the net ionic equation for the reaction that takes place between a solution of magnesium chloride and a solution of sodium hydroxide. B A solution of magnesium nitrate reacts with a.
MgCl 2 aq NaOH aq Mg OH 2 s NaCl aq Both magnesium nitrate and sodium hydroxide solutions are colourless solutions. What is the net ionic equation for the reaction between sodium hydroxide and magnesium chloride. It will be a double displacement reaction leading to the formation of sparingly soluble magnesium hydroxide as a white precipitate.
Mg 2 OH- MgOH2. Sodium chloride exists in. Magnesium nitrate sodium hydroxidesodium carbonate sodium hydroxidesodium hydroxide strontium chloride.
Magnesium hydroxide and hydrochloric acid net ionic equation Magnesium reacts with hydrochloric acid according to the equation. Thus it will not be present in the net ionic equation and are spectator ions. CaCl2aq2AgNO3aq CaNO32aq2AgCl s CaCl 2 a q 2 AgNO 3 a q Ca NO 3 2 a q 2 AgCl s This balanced equation derived in the usual fashion is called a molecular equation because it doesnt explicitly represent the ionic species that are present in solution.
A A solution of potassium hydroxide reacts with a solution of sodium hydrogen phosphate. Sodium hydroxide - diluted solution. A net ionic equation shows only the chemical species that are involved in a reaction while a complete ionic equation also includes the spectator ions.
Ionic form of the above equation follows. Videos you watch may be added to the TVs watch history and influence TV recommendations. Help writing complete ionic and net ionic equations.
The ionic equation of a chemical. Mg 2 OH- MgOH2. Answer 1 of 5.
Write the net ionic equation for Sodium Hydroxide and Magnesium Chloride. Write the net ionic equation for the reaction that takes place between aqueous magnesium chloride and aqueous sodium hydroxide. Hg2NO32 aq CuSO4 --- CuNO32 aq Hg2SO4 s K2CO3 aq MgI2 -- MgCO3 s 2KI 3 NaCrO4 aq 2 AlBr3 --- Al2CrO43 s 6NaBr i got down writing the formula.
Up to 256 cash back Write net ionic equation for the reaction of solutions of sodium hydroxide and magnesium chloride List the Formula equation ionic equation and net ionic equation for the following. Magnesium chloride sodium hydroxide magnesium hydroxide sodium chloride. MgCl 2 2NaOH Mg OH 2 2NaCl.
MgClofaq NaOH aq - MgOH - NaCl aq MgCl aq 2NaOH aq Mg OH 2 NaCl aq Mg aq 2 OHTag Mg OH Mg aq 2Cl aq 2Na aq 2OH aq Mg OH 2 NaCl a Mg aq OH aq MgOH - Question. Write the balanced molecular equation for the reaction including the state of each substance. We can find the net ionic equation for a given reaction using the following steps.
MnCl 2 2NaOH Mn OH 2 2NaCl. Sodium hydroxide - 20 solution. What is the net ionic equation for the reaction between sodium hydroxide and magnesium chloride.
When they react with each other a white precipitate Mg OH 2 and a colourless solution is given. S o d i u m H y d r o x i d e a n d M a g n e s i u m C h l o r i d e. Copper II chloride sodium hydroxide copper II hydroxide sodium chloride.
Manganese II chloride react with sodium hydroxide to produce manganese II hydroxide and sodium chloride. As sodium and chloride ions are present on both the sides of the reaction. Mg s 2 HCl aq â MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single substitution reaction or to demonstrate the generation of hydrogen gas.
This type of reaction is called a precipitation reaction and the solid produced in the reaction is known as the precipitateYou can predict whether a precipitate will form using a list of solubility rules such as those found in the. MgCl2 aq 2NaOH aq MgOH2 s 2NaCl aq The other product sodium chloride remains in solution. This reaction takes place in a nitrogen atmosphere.
There are three main steps for writing the net ionic equation for Mg HCl MgCl2 H2 Magnesium Hydrochloric acid. If playback doesnt begin shortly try restarting your device.

Net Ionic Equation Mgcl2 Naoh Youtube
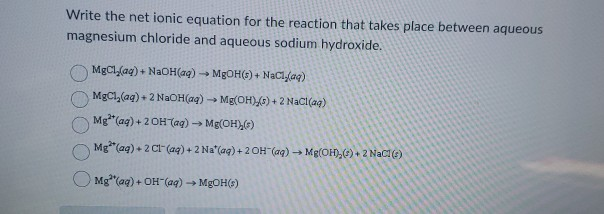
Solved Write The Net Ionic Equation For The Reaction That Chegg Com

How To Write The Net Ionic Equation For Naoh Mgcl2 Nacl Mg Oh 2 Youtube

How To Write The Net Ionic Equation For Naoh Mgcl2 Nacl Mg Oh 2 Youtube
Comments
Post a Comment